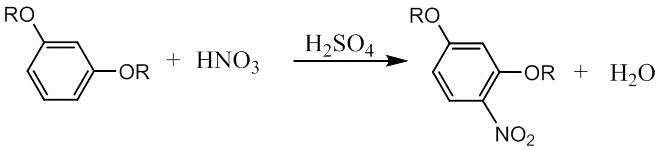
Nitrification
involves introducing a carbon atom of an organic substance into a nitro group
to increase the activity of a nucleophilic displacement reaction, or converting
a nitro group into another group to prepare for the next reaction. The
nitrification reaction is exothermic and the by-products are formed when the
reaction time is too long. Therefore, reasonable control of the temperature and
time of the reaction is the key to success.
Himile
used microchannel reactor to conduct a small test on the nitrification
synthesis reaction of pendimethalin.
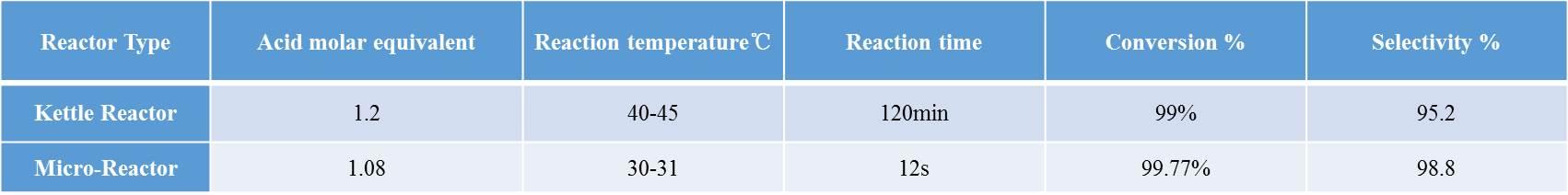
The
data resulted from conventional tank reactors and microchannel reactors are
listed in the table below:
The
small-scale experimental experiment with the microchannel reactor solves the
problems of the traditional tank reactor, such as intense exothermation, long
reaction time and many by-products, reducing the amount of nitric acid and
impurities, and improving the reaction conversion and selectivity. The batch
process is converted to a continuous process to improve product stability.